Navigating the world of biosimilars for better health outcomes and cost-effective care
- Biosimilars are the more affordable biologics with no clinically meaningful differences in purity, efficacy, and safety with respect to reference biologics.1
- Their regulatory approval follows strict standards of quality, safety, and efficacy as is common with other therapeutics.1,3
- By embracing biosimilars, the healthcare system can navigate towards a future of more accessible, efficient, and affordable care for patients.3
Biologics, or biological products, encompass a variety of therapeutic agents, including blood, blood components, vaccines, allergenics, cells, gene therapies, and recombinant therapeutic proteins. These products are derived from living organisms such as animals, humans, and microorganisms. Biologics are produced using advanced biotechnological methods and represent significant advancements in the biomedical field, offering treatment for complex medical conditions that previously had no available treatment options.4
Biologics to biosimilars – unveiling new horizons
Because they are made from living cells, biologics are more structurally and functionally complex than typical small-molecule drugs. Developing and manufacturing biologics require cutting-edge biomedical research and advanced biotechnological methods resulting in their high cost.5 For instance, biologics make up only 2% of all US prescriptions but comprise 40% of the total spending on prescriptions.3 This poses a significant economic burden on healthcare systems, constraining resources and restricting access to patients. More so, in developing countries with fragmented public-funded healthcare, patients often cannot afford the best available treatment option offered through biologics due to its expensive nature.6,7
The loss of market exclusivity for biologics has led to the development of biosimilars, the non-innovator versions of the reference biologic drug, defined by various regulatory bodies as described in Table 1.5,8 It has led to a more accessible and equitable treatment landscape increasing the treatment access and prescription choice for patients and physicians, respectively.7,9
Table 1: Definition of biosimilars by major regulatory authorities worldwide8
| Regulatory Guidelines | Definition |
| European Medicines Agency (EMA) | A biologic medicinal product similar to another biologic medicine that has already been authorized for use |
| World Health Organization (WHO) | A biotherapeutic product that is similar in terms of quality, safety, and efficacy to an already licensed reference biotherapeutic product |
| United States Food & Drug Administration (US FDA) | A biologic product that is highly similar to the reference product notwithstanding minor differences in clinically inactive components and that there are no clinically meaningful differences between the biologic product and the reference product in terms of safety, purity, and potency of the product |
| Pharmaceuticals and Medical Devices Agency (PMDA) | A biotechnological drug product developed by a different company, which is comparable with an approved biotechnology-derived product |
Low-to-middle-income countries carry a high burden of non-communicable diseases as well as healthcare disparities. Biosimilars are important in addressing healthcare disparities by expanding access to life-saving medicines.10 The World Health Organization acknowledges health as a basic human right and asserts that everyone should have access to health services without facing financial difficulties.11 Biosimilars are a step in this direction that can help in bringing health equity to everyone.
Understanding biosimilars – the development and approval pathways
Biosimilars are approved through the same pharmaceutical quality, safety, and efficacy standards applied to other biologics. Due to the inherent variability associated with biopharmaceutical manufacturing, biosimilars are not the exact copy of their reference biologic. Instead, the biosimilar development program aims to demonstrate high similarity in structure, function, efficacy, safety, and immunogenicity through extensive pre-clinical and clinical comparative analysis. The key stages in the original biologic and biosimilar development program are described in Figure 1.1 As can be easily gauged, biosimilars do not undergo the clinical research program at the scale required for reference medicine, but the bulk of studies are about establishing molecular and structural similarity. Biosimilars should show no clinically meaningful differences from the reference molecule, and any variability should be kept within strict limits.1
Biosimilars leverage the safety and efficacy data of the reference medicine by demonstrating comparable safety and efficacy in one indication, which may be extrapolated to all the other approved indications of the reference medicine. This helps in circumventing repetitive multilevel assessment through clinical trials for each new indication resulting in a shorter development program and cost-effectiveness. This concept of extrapolation is rooted in scientific principles and is routinely used for approved biologics if they undergo any major manufacturing changes, not requiring repeat clinical trials for all indications.12
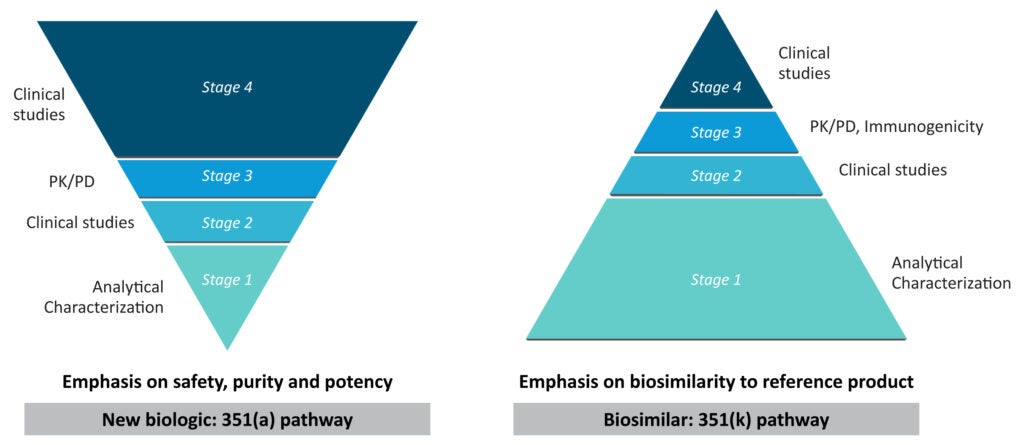
Figure 1: Comparing the development pathways for new biologic and biosimilars
(The figure is for illustration purposes only and is adapted from Sheridan et al. 2024).1
European Union was the first to establish a biosimilar regulatory approval framework and based on it European Medical Agency (EMA) approved the first biosimilar in 2006.12 In 2009, the Food and Drug Administration (FDA), USA rolled out the 351(k) pathway, a streamlined process for approval of biosimilars.1 Europe currently leads the way in biosimilar acceptance representing 60% of the global biosimilar consumption.9 It is important to highlight that in its 10 years of experience with biosimilars, the EU monitoring system for safety concerns has not documented any significant safety concerns for biosimilars with no relevant differences in the nature, severity, or frequency of any adverse events.12
Using biosimilars to increase access and reduce cost
The availability of biosimilars as a cost-effective alternative to reference biologics offers a promising avenue to reduce healthcare costs and enhance access to treatment. As per an IQVIA report, between 2016-21, the total European savings at list prices as a direct impact of biosimilar competition is estimated to be about €50 billion.13 It is estimated that switching to infliximab biosimilar, a TNFα inhibitor used for treating rheumatoid arthritis, could result in about € 233-433 million saved over 5 years in the UK, France, and Germany alone. This roughly translates to a 20-30% discount on the current selling price of infliximab. These savings can be further utilized to treat roughly 7500 additional rheumatoid arthritis patients.3 Similarly, in the US, biosimilars have resulted in an estimated savings of $13 billion since 2015 as their prices are typically 15-35% lower than the respective reference biologic and are expected to save between $38-124 billion from 2021-2025.14 Higher utilization of rituximab biosimilar prescribed for cancer and rheumatoid arthritis treatment can bring savings in the range of 44-69% on the originator’s selling price with roughly 12% lower out-of-pocket expenses for patients.3 The competition due to biosimilars were reported to have reduced the costs for Medicare Part B and enrollees.10
This potential reduction in costs is pivotal in enhancing patient access to biologic treatments.15 This improved affordability can enhance patient adherence, particularly for those who might otherwise struggle to afford their medications, especially in emerging economies with poor healthcare infrastructure.10 For example, Southeast Asian countries like Malaysia have seen significant price drops (up to 40%) and a corresponding increase in access to insulin (about 30%) since the launch of their biosimilars in 2011.10 India has a booming biosimilar landscape and had recognized its value in affordable healthcare far earlier, approving the first biosimilar in year 2000 and formally adopting biosimilar regulatory guidelines in 2012.7
Biosimilars – a battle of perception and utilization
As discussed above biosimilars have no clinically meaningful differences in structure and function with respect to reference biologics and are approved through stringent regulatory processes that ensure their quality, clinical efficacy, and safety.12 There is significant evidence that starting with biosimilars or transition from bio-originators to biosimilars product to a biosimilar does not diminish treatment efficacy or raise the likelihood of adverse events.1
Despite the rise in regulatory approvals and cost-saving potential, multiple barriers deter biosimilar uptake.16 Biosimilar adoption in clinical practice largely depends on policies that promote uptake, as well as healthcare professionals’ and patients’ understanding of biosimilars and confidence in their use.14 Trust from prescribers is crucial for patients to embrace biosimilars and lower the occurrence of the nocebo effect (negative expectations of the patient regarding a treatment cause the treatment to have a more negative effect than it otherwise would have).1 An increased understanding and education about these alternatives are essential for integrating them into clinical practice.,15 To learn more about the policies that can enhance biosimilar uptake, please read our article, Policies and practices in healthcare system to promote biosimilar use.
Biosimilars for improved adherence
Reports have suggested that the higher cost of biologics leads to lower adherence over time. Biosimilars offer a more cost-effective alternative to high-cost reference biologics and therefore could positively impact medication adherence.15 In a study on non-physician-administered biologics, higher copayments were associated with lower rates of adherence.17
A nationwide cross-sectional study in France on patients with rheumatic inflammatory disease revealed that being informed about biosimilars and a good understanding of the definition of biosimilars were characteristics associated with better adherence to biosimilars.18 This highlights the potential of biosimilars in not just enhancing the affordability but also in promoting sustained therapeutic adherence.
Conclusion
In the dynamic healthcare sector, biosimilars stand out for making critical treatments more accessible and financially bearable.1 Regulatory frameworks have streamlined their entry, maintaining high standards for similarity and effectiveness, thus preserving therapeutic integrity.12 Europe leads the way in biosimilar approvals and consumption (almost 60% of the global consumption), setting a path that others can follow to reduce healthcare costs and democratize access to essential medications.9 As low-to-middle-income countries are slowly pacing up with regulatory approvals for biosimilars, they also need to focus on policies that would enhance its uptake. Finally, the effort should not only be about cutting costs, but also about impacting patient outcomes and enhancing adherence.
“The essence of global health equity is the idea that something so precious as health might be viewed as a right?”
– Paul Farmer, an American anthropologist and physician













