Monitoring medication adherence is critical: A sneak peek into latest technologies.
- Monitoring adherence is a challenging task. The direct methods of monitoring are effective but cumbersome leading to reliance on indirect methods of adherence assessment 1.
- Advances in technology have led to the development of electronic adherence monitoring devices that offer higher accuracy, convenience, and affordability as well as allow continuous monitoring of adherence behavior 1,2.
- Employing these devices may result in a significant positive impact on medication adherence3. Nevertheless, balancing technological solutions with human interaction is the optimal method of patient care 1-3.
H. James Harrington, a pioneer in performance improvement, once said – “Measurement is the first step that leads to control and eventually to improvement. If you can’t measure something, you can’t understand it. If you can’t understand it, you can’t control it. If you can’t control it, you can’t improve it.” Monitoring and evaluation are two pillars for assessing the performance and the impact of any intervention. Monitoring is defined as a continuum functionality that provides the progress of an intervention or lack thereof, in achieving a result 4.
Assessing treatment efficacy in clinical practice or research settings should consider evaluating and monitoring medication adherence, yet it remains a challenging issue 2. There is no “gold standard” of monitoring adherence and each method has its advantages and limitations. Therefore, the utility of advanced technology in monitoring systems is being recognized for its potential to enhance medication adherence, especially for long-term treatment regimens 2.
In one of our previous articles, “Measuring adherence – an Achilles heel in medication adherence”, we discussed the importance and challenges of measuring adherence emphasizing the benefits of electronic monitoring systems. The present article discusses the latest in medication adherence monitoring technologies, their advantages and impact on medication adherence as well as debates their potential to replace the human touch.
Monitoring adherence: Far easier said than done
Multiple methods exist for the assessment of medication adherence, each has its advantages and pitfalls. Broadly, they can be categorized into two groups one that allows direct measurement and others that are indirect methods using proxies for assessment 1.
Direct methods include directly observed therapy (DOT), biomarkers or drug metabolites assessment in biological fluids, and electronic monitoring of medication uptake through videos, sensors, wearables, etc. Rather, indirect methods use pill counts, pharmacy refill records, and patient self-reports to estimate treatment adherence 1,2. Self-reporting has also gained technological advancements such as eDiaries, web-based platforms, and mobile applications to provide real-time, albeit subjective, medication adherence data 2.
It can be appreciated that the direct methods provide a more objective and accurate assessment of medication adherence, whilst the indirect methods are largely estimates of treatment adherence and are subjective 1,5
Electronic monitoring systems: Monitoring from a distance
Although direct methods for measuring adherence are desirable, they are more demanding on time and effort1. The development of electronic medication monitoring systems is a game changer in medication adherence monitoring. These electronic devices used advanced technologies to record the time and date of the medication removal event from the respective device and relay it in real-time to healthcare professionals 2.
- Medication Event Monitoring Systems (MEMS): These devices include electronic pill bottles, bags, or boxes varying in their physical appearance as well as the number and types of medications they can store. Such devices may contain a sensor that can record every time the patient opens the pill bottle. The data can also be relayed in real-time to practitioners alerting them in case of multiple missed doses. Even though it is not a direct method of adherence measurement, it provides objective medication adherence monitoring that may be both convenient and acceptable to patients. However, actions, like opening the bottle and not taking the pill, taking out more than one pill, or taking out the pill but not ingesting, can cause inaccuracy in the data. Other types of MEMS include blister packaging and electronic inhalers. Both can document the date and time of the medication-taking event as well as relay the information in real-time. However, it cannot ensure the medication was ingested/inhaled. A few new electronic inhaler devices can record the sound of inhalation to provide a better assessment of adherence 2,6.
- Electronic Medication Management Systems (EMMS): These include a group of devices that helps patients manage their often-complex medication regimens and document the medication-taking event which can be further relayed to HCPs in real-time. One of the latest EMMS devices includes RFID-enabled medication adherence intelligence systems (RMAIS). These are an RFID (radio frequency identification)-based medication dispensing system that enables correct dispensing of multiple medications stored in individual RFID-labelled bottles. At a preset time, the system sounds an alarm and dispenses medication only from the correct bottle. The medication removal is automatically documented by the system through the reduction in the weight of the medication bottle before and after dispensing 2.
All the above-mentioned technologies use proxy methods to assess adherence such as opening the lid or weight of the medication bottle, leaving a scope for human error causing inaccuracy in the adherence data2.
There is a need for advanced technologies for monitoring and measuring medication adherence through more direct methods. Usually, direct methods such as DOT or assessment of biological markers are more time-consuming requiring extra effort and less patient friendly. Therefore, the newer technologies for direct adherence monitoring should be less cumbersome, time-consuming, and more patient-friendly.
What’s “a la mode” in direct monitoring technologies?
Recent advances in technology have led to the development of innovative systems that can measure and track patients’ medication adherence in real-time and offer the advantage of direct monitoring. A few examples of such systems include ingestible sensors, video-based technology, and motion sensor technology2. The defining features of these monitoring systems, their benefits, and limitations are discussed below:
- Digital pills:These are innovative sensors embedded in the pills that can monitor medication adherence directly in a remote way. Microsensors-enabled pills and a monitor worn externally on the abdomen along with a mobile app make up the technological framework of these ingestible sensors. Once the pills are ingested, the sensor signals to the external monitor which then records and relays the information to HCPs in real-time using the mobile app. The device is also used for remotely monitoring other physiological parameters. It has been reported to be feasible for a wide range of therapeutic areas 2,7.
- Video-based monitoring technology:These technologies enable remote yet direct observation of medication uptake through video recording called video DOT or V-DOT2. This has been a cornerstone for diseases like tuberculosis where in-person observation is considered the gold standard. In this case, Video-DOT was reported to produce comparable disease outcomes as DOT while using the resources more efficiently and is now recommended as an equivalent alternative to DOT 8.
- Motion sensor technology:These are neck- or wrist-based wearables that use accelerometers and motion-sensing gyrometers that can sense the hand movements made by patients to ingest the medication. This data is documented and shared with care providers in real-time. However, it does mistake other hand movements like drinking water or wiping the nose with medication uptake activity resulting in inaccuracy of the data 2.
- Medication Behavior Monitoring Systems (MBMS): This system uses several advanced technologies like artificial intelligence, deep learning, motion-sensing, electronic pillboxes, and video monitoring. The device sounds an alarm at a pre-set time and senses the patient’s movement towards itself. Once the patient is close by, it starts video-recording the event. The device dispenses the medication on a scale once it senses the arm-raising movement for drinking water and documents the event positively when the pill is taken up from scale2.
- ReX: This is an advanced hand-held medication dispensing unit that is supported remotely by a care team through cloud-based management. It comprises a reusable dispensing unit that gives timely reminders and stores medication along with a disposable cassette that delivers the medicine as per the preprogrammed treatment protocol directly to the patient’s mouth. It provides real-time adherence data to the care team and also asks customized clinical questions to the patients to enable remote monitoring by the care team 2,9.
Electronic devices are the way forward in monitoring adherence
Resource-intensive interventions like direct methods for monitoring adherence are not sustainable in the long-term1. Electronic devices that provide remote real-time measures of adherence may be beneficial as they require less time, effort, and cost plus, they can deliver more accurate information. The main benefits of these electronic monitoring devices are mentioned in Figure 1.
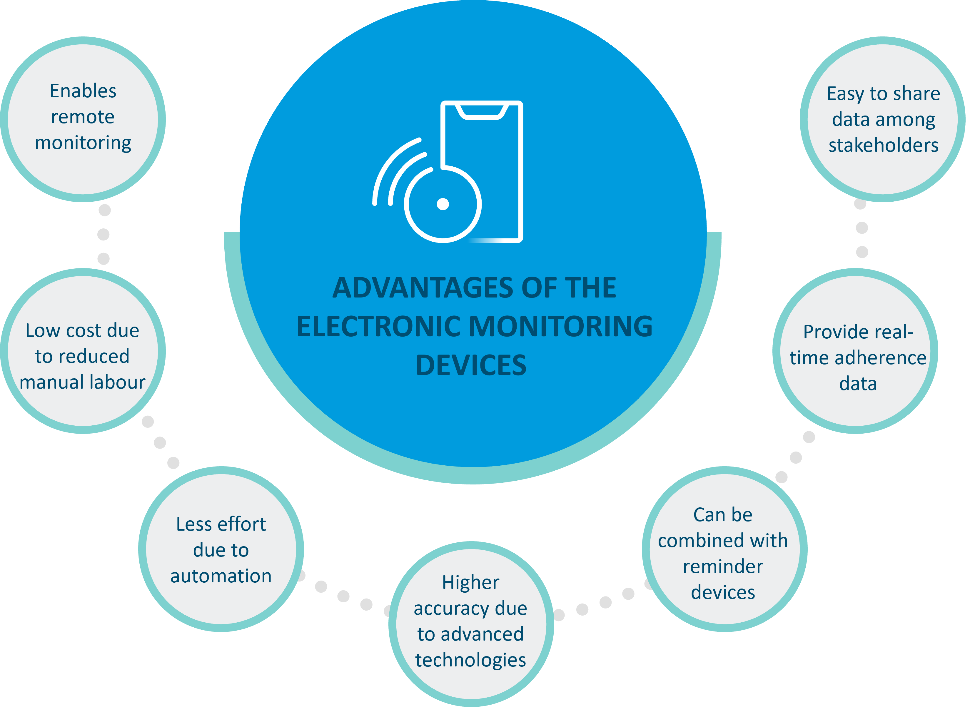
Figure 1: Various advantages of using electronic devices for monitoring medication adherence 1,2.(This image was created for illustrative purposes and is based on data obtained from Aldeer et al., 2022, and Mason et al., 2022).
Nevertheless, limitations in using these devices should also be acknowledged such as data accuracy, energy consumption, data fidelity, and patient comfort 1,2.
Impact of electronic adherence monitoring
Electronic adherence monitoring devices may be considered standard due to their objectivity and relative data accuracy as a convenient option for both patients and care providers. Employing these devices has led to comparable or even superior outcomes in terms of treatment completion and adherence when compared to in-person DOT as seen in multiple therapeutic areas across several studies 10-12.
A systematic review analyzing the impact of electronic adherence monitoring (EAM) devices on medication adherence and treatment outcomes reported significantly better adherence as compared to control (standard mean difference: 0.93; 95% CI, 0.69-1.17) across various chronic conditions 3.
Electronic monitoring devices – can they replace the human touch?
Although not without limitations, the usage of electronic monitoring devices offers viable and effective alternatives to traditional in-person methods. However, there will always be a need for human touch in healthcare, which includes empathy, support, and individual care. The optimal method of patient care can only be achieved by combining the efficiency and precision of EAM technology with inputs from healthcare providers and patients1-3. Achieving the ideal balance between innovative technology and human connection is crucial for providing patient-centered care.
“Technology can assist a qualified well-trained human being, cannot replace him”
– Isaac Yeffet, an expert in aviation security from Israel.












